
ABSTRACT
Aromatic oligomers constitute a distinct and promising class of synthetic foldamers – oligomers that adopt stable folded conformations. Single helical structures are, to a large extent, predictable, show unprecedented conformational stability in essentially all solvents, and represent convenient building blocks to elaborate synthetic, very large (protein-sized) folded architectures. Solution phase synthesis gives access to aromatic amino acid monomers bearing all kinds of biogenic-like side chains,[1] and solid phase synthesis of long oligomers has been automated. [2] In some instances, protein-sized foldamers can even self-synthesized, that is, spontaneously emerge from their precursor monomer.[3] This lecture will highlight how these sizeable folded molecules can be designed and used to recognize proteins, a challenge that can be addressed through different approaches, i.e. biomimicry,[4] screening[5] or computational design and combinations thereof.[6]
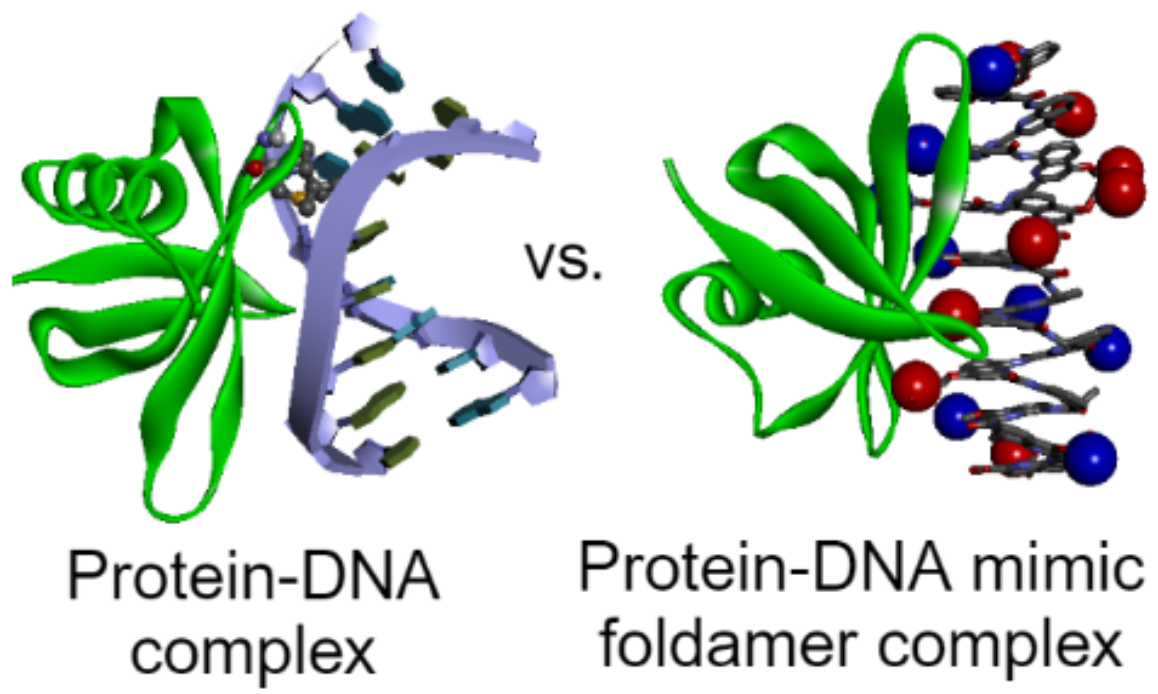
Figure 1. Comparison of the crystal structures of a small chromosomal protein in complex with DNA (left) or with a foldamer that mimics DNA (right).[4a]
References
[1] M. Zwillinger, P. Sőregi, F. Sanchez, C. Douat, M. Csékei, I. Huc, A. Kotschy, J. Org. Chem. 2025, 90, 3043.
[2] V. Corvaglia, F. Sanchez, F. S. Menke, C. Douat, I. Huc, Chem. Eur. J. 2023, 29, e202300898
[3] C. G. Pappas , P. K. Mandal , B. Liu, B. Kauffmann , X. Miao, D. Komáromy , W. Hoffmann , C. Manz, R. Chang, K. Liu , K. Pagel , I. Huc, S. Otto, Nat. Chem. 2020, 12, 1180.
[4] (a) D. Deepak, J. Wu, V. Corvaglia, L. Allmendinger, M. Scheckenbach, P. Tinnefeld, I. Huc, Angew. Chem. Int. Ed. 2025, 64, e202422958. (b) K. Ziach, C. Chollet, V. Parissi, P. Prabhakaran, M. Marchivie, V. Corvaglia, P. P. Bose, K. Laxmi-Reddy, F. Godde, J.-M. Schmitter, S. Chaignepain, P. Pourquier, I. Huc, Nat. Chem. 2018, 10, 511.
[5] (a) S. Dengler, R. Howard, V. Morozov, C. Tsiamantas, Z. Liu, C. Dobrzanski, V. Pophristic, S. Brameyer, C. Douat, H. Suga, I. Huc, Angew. Chem. Int. Ed. 2023, 62, e202308408. (b) S. Kwon, V. Morozov, L. Wang, P. K. Mandal, S. Chaignepain, C. Douat, I. Huc, Org. Biomol. Chem. 2024, 22, 9342.
[6] L. Wang, C. Douat, J. Sigl, S. Reddy Post, L. Fischer, B. Langlois d’Estaintot, Z. Liu, V. Pophristic, Y. Yang, Y. Zhang, I. Huc, Chem. Sci. 2025, 16, 12385.